Roles and Responsibilities under ICH GCP
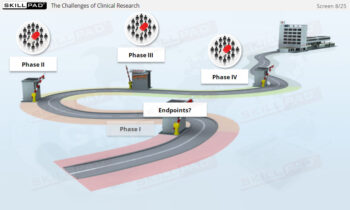
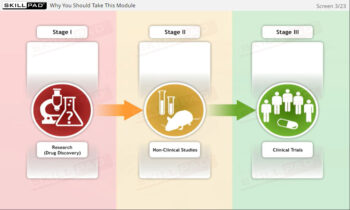
An exploration of the principles and guidelines outlined in ICH Good Clinical Practice (GCP), focusing on the ethical and scientific standards necessary for conducting clinical trials. This module covers the roles of key parties involved in clinical trials, including sponsors, investigators, and Institutional Review Boards (IRBs), with an emphasis on the importance of informed consent. The module also details the trial protocol, a crucial document that guides clinical trial design and execution, ensuring both the protection of trial subjects and the integrity of the trial data.
Whether for onboarding or annual refresher training, this Module is seamlessly deployable on any LMS and can be tailored to your company’s exact needs.
Duration: 30 Mins
Grade: Core Library
Description
- Gain Insight into GCP Principles: Examine the ethical standards and scientific integrity requirements of clinical trials to ensure the credibility and safety of trial data.
- Recognize Roles and Responsibilities: Explore the responsibilities of sponsors, investigators, and Institutional Review Boards (IRBs) in conducting ethical and compliant clinical trials.
- Understand Consent: Gain a clear understanding of the informed consent process, ensuring that trial subjects are well-informed, and their rights and safety are prioritized.
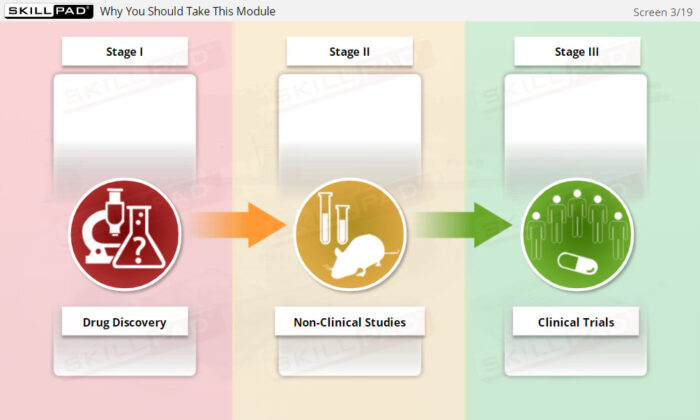
- Follow Protocol and Maintain Compliance: Understand the role of the clinical trial protocol, including its design, content, and the steps required to meet regulatory standards and GCP guidelines.
Learning Objectives
- Understand and explain at an overview level the meaning of the term: Good Clinical Practices (GCP).
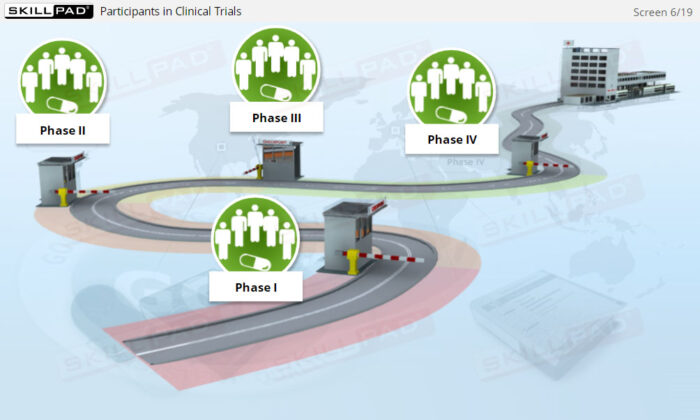
- Describe the role and rights of participants in clinical trials.
- Describe the roles and responsibilities of sponsors, investigators and IRB/IECs in clinical trials.
- List the other key contributors in clinical trials.
- Explain the principle of informed consent.
- Describe the purpose, contents and development of the ‘Trial Protocol’.
Keywords
- Clinical Trials
- Clinical Trial Data
- Clinical Trial Protocol
- Good Clinical Practice (GCP)
- Human Subjects Protection
- ICH GCP
- Independent Ethics Committee (IEC)
- Informed Consent
- Institutional Review Board (IRB)
- Sponsors
Module Features
Animations
Voice Over
Knowledge Checks
Assessments
SCORM/AICC compatible
Full Screen