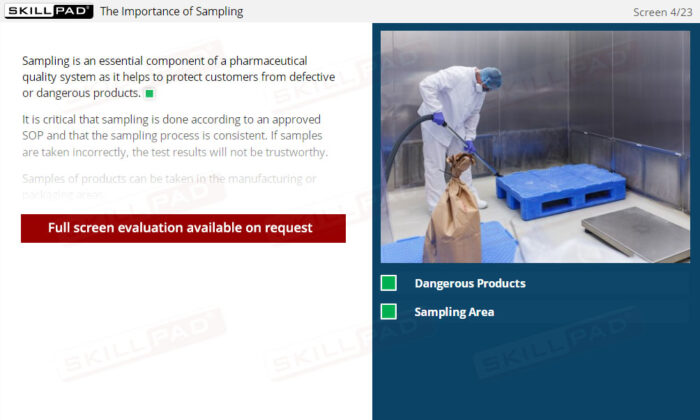
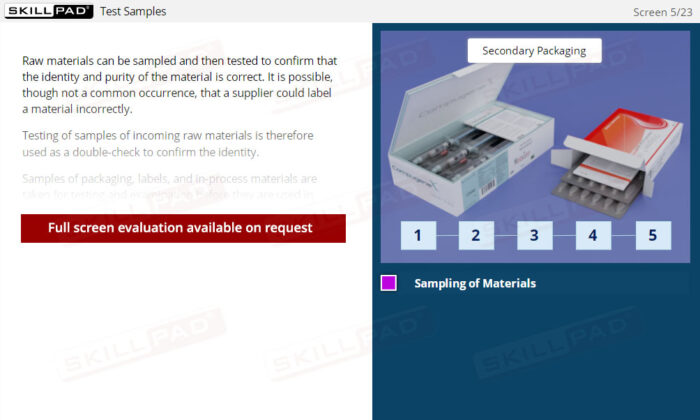
Sampling
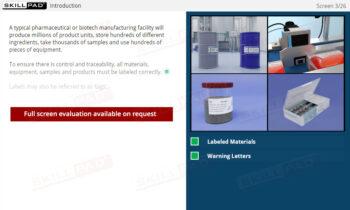
An overview of the critical role that sampling plays in pharmaceutical and biopharmaceutical manufacturing. This module explains the principles and procedures for collecting representative samples from raw materials, in-process materials, and final products to ensure quality, safety, and compliance with regulatory standards. Learners will explore best practices for sample container selection, proper labeling, and adherence to Standard Operating Procedures (SOPs) to minimize the risk of contamination or erroneous test results. The module also covers the distinctions between reserve and stability samples and the importance of correct sampling techniques to prevent potential product quality issues.
Whether for onboarding or annual refresher training, this Module is seamlessly deployable on any LMS and can be tailored to your company’s exact needs.
Duration: 30 Mins
Grade: Core Library
Description
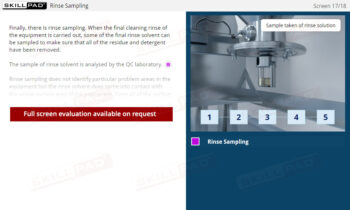
- Learn About Sampling Techniques: Understand the importance of representative sampling and its impact on product quality and consumer safety. Learn the procedures for collecting and handling samples accurately to ensure reliable testing outcomes.
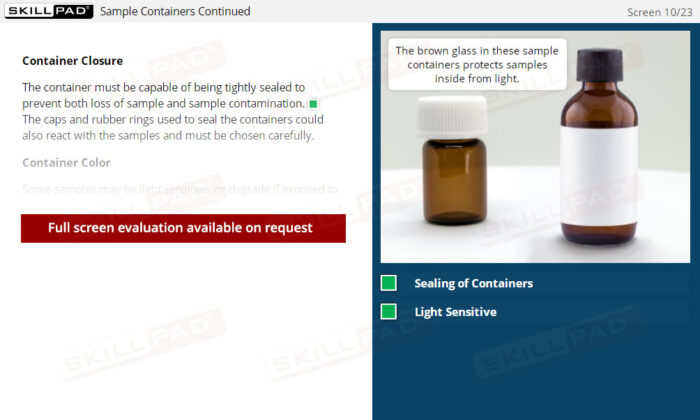
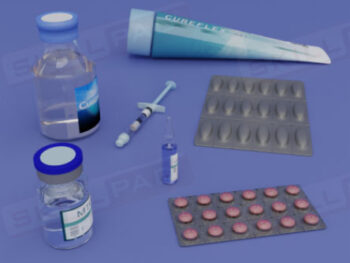
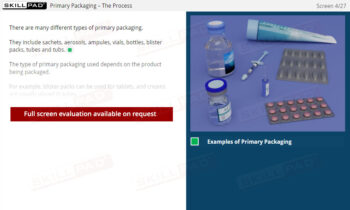
- Understand Sample Container Selection and Use: Gain knowledge of the characteristics of appropriate sample containers, including considerations for material, closure, and color, to prevent contamination or degradation.
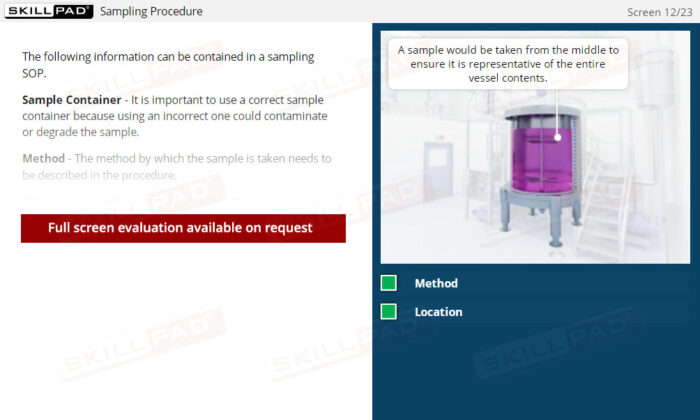
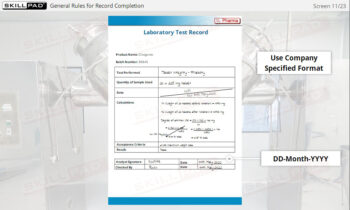
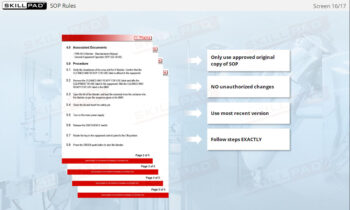
- Learn About Sampling SOPs: Understand the key elements of a well-designed Sampling SOP, including methods, sampling locations, and equipment requirements, to consistently collect and store high-quality samples.
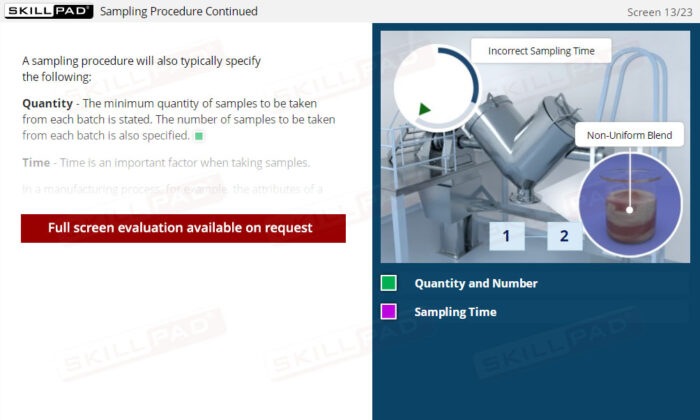
- Recognize Sampling Errors and Their Impact: Learn about the potential consequences of incorrect sampling, such as product recalls or safety risks, and how to prevent these errors through proper training and adherence to protocols.
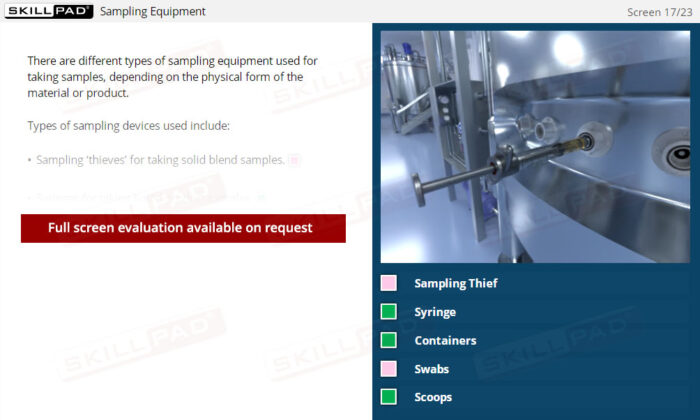
- Learn About Specialized Sampling Equipment: Become familiar with different sampling tools tailored to various material forms and understand their correct use to maintain safety and accuracy.
Learning Objectives
- Explain why sampling is done in pharmaceutical manufacturing facilities.
- Name the types of samples that are typically taken.
- Describe the attributes that a sample container should have.
- List the important information that should be contained in a sampling SOP.
- Explain the terms ‘Reserve Sample’ and ‘Stability Sample’.
- Describe the information that should be contained on a sample label.
- Describe the consequences of taking samples incorrectly.
Keywords
- Active Ingredient
- Batch Integrity
- Contamination Prevention
- GMP Standards
- Product Sampling
- Quality Control
- Reserve Samples
- Sampling Techniques
- Standard Operating Procedures (SOPs)
Module Features
Animations
Voice Over
Knowledge Checks
Assessments
SCORM/AICC compatible
Full Screen