Serialization and Product Tracking
An overview of serialization and product tracking technologies, focusing on their critical role in the pharmaceutical and biologics industries. This module explains how serialization provides a unique identifier for each product, enabling full traceability from production to patient. It delves into the regulatory landscape surrounding serialization, explores key terms like “track and trace” and “authentication,” and outlines the steps involved in implementing a serialization program. With a particular emphasis on production line integration and GS1 standards, this module helps learners understand how serialization ensures product integrity and combats counterfeiting in the global supply chain.
Whether for onboarding or annual refresher training, this Module is seamlessly deployable on any LMS and can be tailored to your company’s exact needs.
Duration: 30 Mins
Grade: Premium Performance
Description
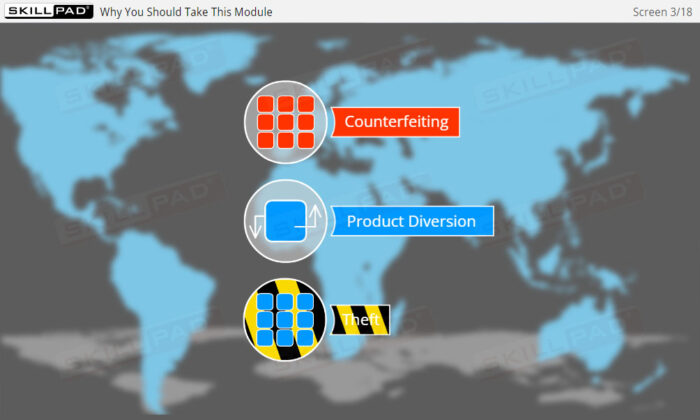
- Understanding Serialization and Its Importance: Gain a comprehensive understanding of serialization and product tracking, including their role in preventing counterfeit drugs and ensuring patient safety across the pharmaceutical supply chain.
- Knowledge of Regulatory Requirements: Learn about the various global regulations that mandate serialization and product tracking, and how they impact the lifecycle of medicines from production to distribution.
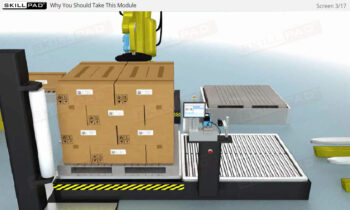
- Practical Implementation Insights: Learn the stages of implementing a serialization program, from strategic planning and solution design to validation and scaling, ensuring smooth integration into existing packaging lines.
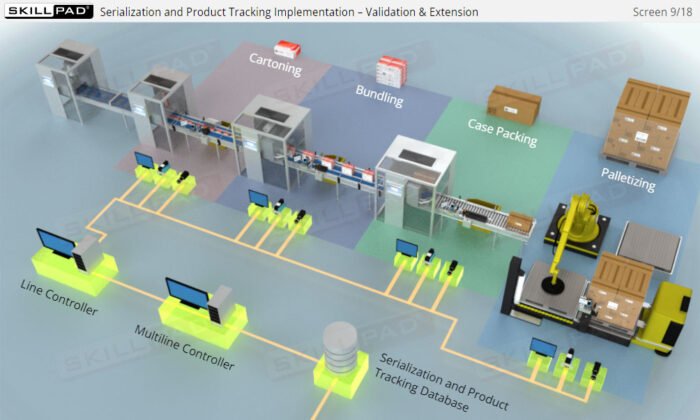
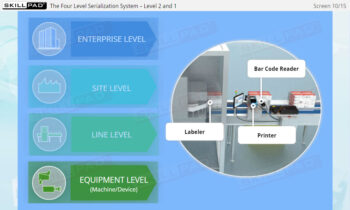
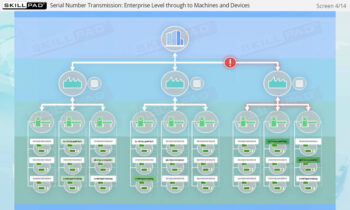
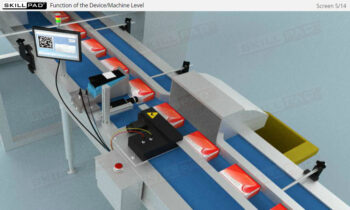
- Familiarity with Production Line Integration: Understand how serialization technologies are incorporated into production lines, including the required equipment and system upgrades necessary for compliance with serialization standards.
- Mastery of GS1 Standards: Understand the significance of GS1 standards for global traceability, ensuring consistency and compatibility across the supply chain for smoother international operations and regulatory compliance.
Learning Objectives
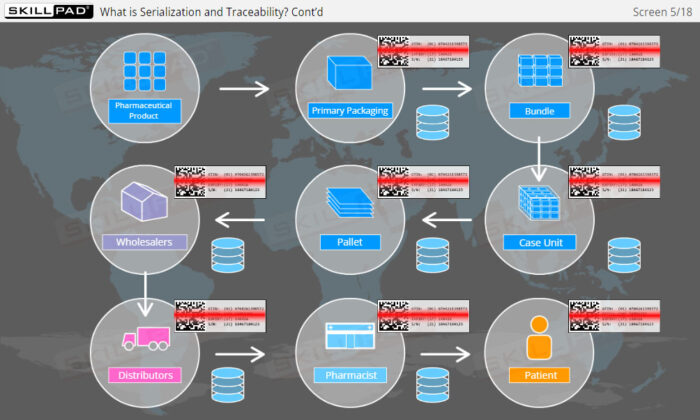
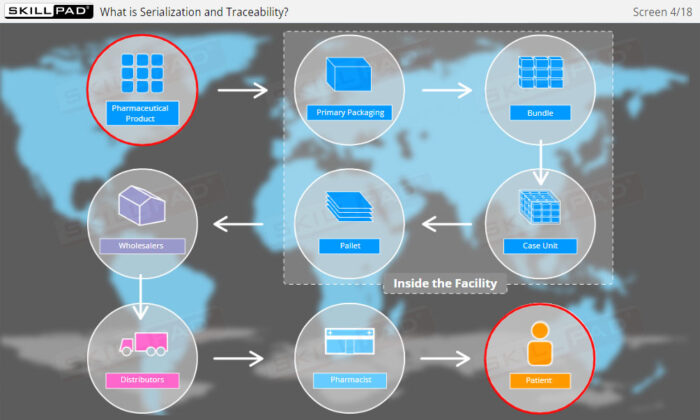
- Explain what is meant by ‘Serialization and Product Tracking’.
- Describe the regulatory background to serialization and product tracking.
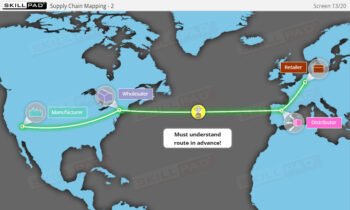
- Explain why serialization and product tracking of medicines is needed.
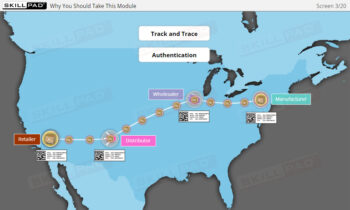
- Explain the terms ‘Track and Trace’ and ‘Authentication’.
- List the typical components of a packaging line that is configured for serialization of prescription drug products.
- Summarize the stages involved in implementing a serialization and product tracking program.
Keywords
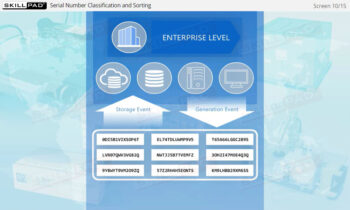
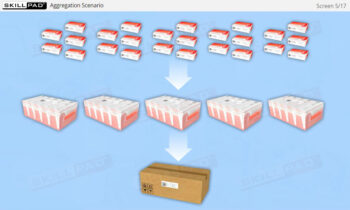
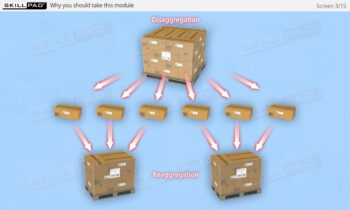
- Aggregation
- Authentication
- Barcodes
- Counterfeit
- Distribution
- Drug Authentication
- Counterfeiting
- Electronic Tagging
- GS1 Standards
- Global Supply Chain (GSC)
- Item Level Serialization
- Product Lifecycle
- Product Tracking
- Regulatory Compliance
- RFID
- Serialization
- Serialization Program
- Supply Chain
- Track and Trace
- Validation
Module Features
Animations
Voice Over
Knowledge Checks
Assessments
SCORM/AICC compatible
Full Screen