Serialization and the Supply Chain
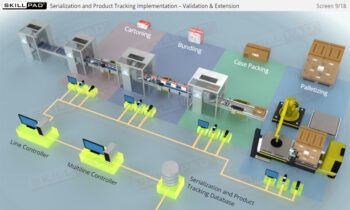
An overview of the serialization and product tracking processes as they pertain to the pharmaceutical supply chain, focusing on the key stages that occur once serialized products leave a regulated production facility. The module covers the regulatory framework, including the Drug Supply Chain Security Act (DSCSA), and explains the necessary steps for achieving compliance with serialization requirements. Learners will explore the concepts of “Track and Trace” and “Authentication,” as well as the challenges faced when products change ownership within the supply chain. Emphasis is placed on the critical manual operations involved in serialization, such as disaggregation, rework, and product recall.
Whether for onboarding or annual refresher training, this Module is seamlessly deployable on any LMS and can be tailored to your company’s exact needs.
Duration: 30 Mins
Grade: Premium Performance
Description
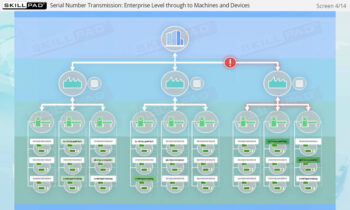
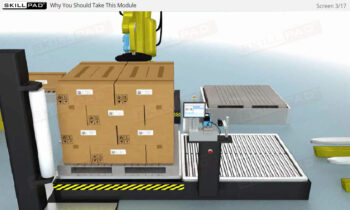
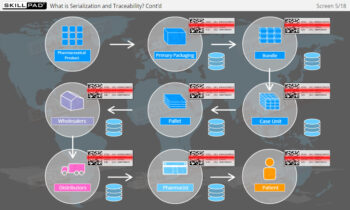
- Comprehensive Understanding of Serialization Processes: Learn the full scope of serialization and product tracking, from initial packaging and serial number generation to movement through wholesalers, distributors, and dispensers, all the way to the end user.
- Knowledge of Regulatory Compliance: Gain insight into the Drug Supply Chain Security Act (DSCSA) and its impact on serialization practices. Understand how to ensure compliance with regulatory standards related to the traceability of drug products.
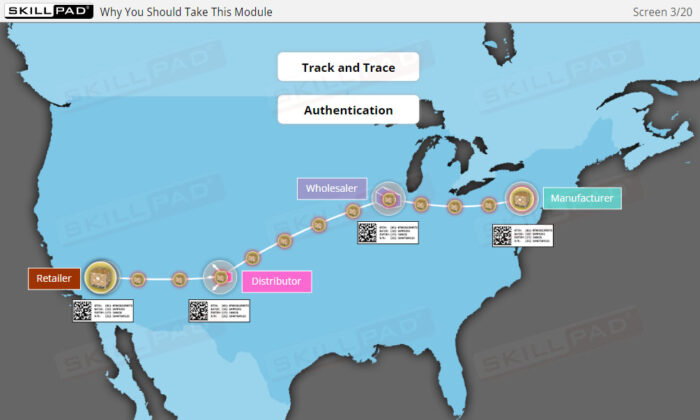
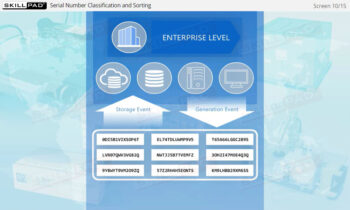
- Expertise in Track and Trace Systems: Master the concepts of “Track” and “Trace,” and learn how these systems ensure accurate product tracking across the supply chain, addressing both current location and historical movements.
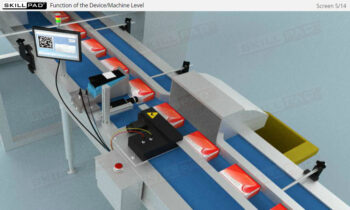
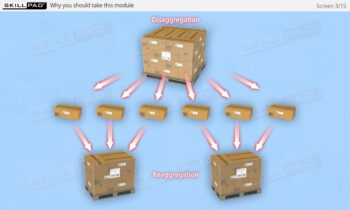
- Practical Insights into Manual Serialization Operations: Understand the manual operations critical to maintaining product integrity, including the decommissioning of serial numbers, product disaggregation, and handling situations like product recalls or suspected tampering.
- In-depth Knowledge of Ownership Transitions in the Supply Chain: Explore the complexities of ownership transfer within the pharmaceutical supply chain, including challenges and best practices for tracking changes in ownership from manufacturer to dispenser.
Learning Objectives
- Outline key aspects of the Drug Supply Chain Security Act (DSCSA) and the European Union Falsified Medicines Directive (EUFMD).
- Define the terms: “Transaction Information”, “Transaction History”, and “Transaction Statement”. (Also known as “TI / TH / TS”)
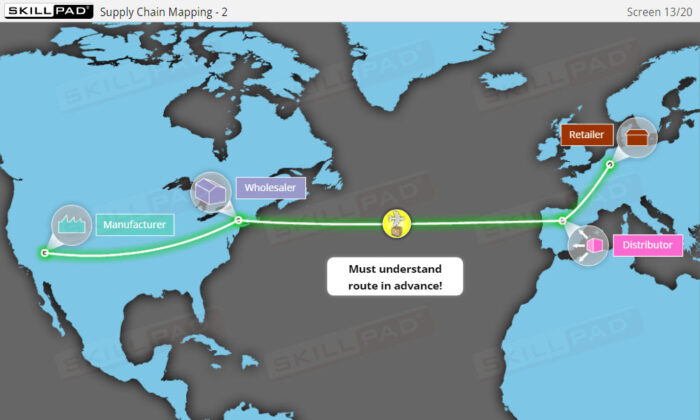
- Describe key aspects of Supply chain dynamics such as ownership, supply chain mapping and ‘Free on Board’.
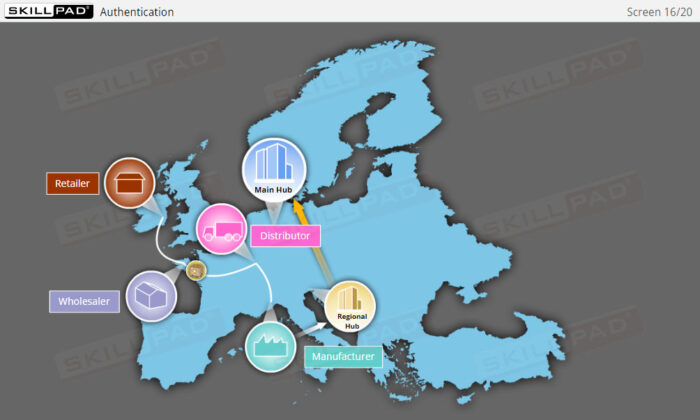
- Explain the difference between ‘authentication’ and ‘track & trace’.
Keywords
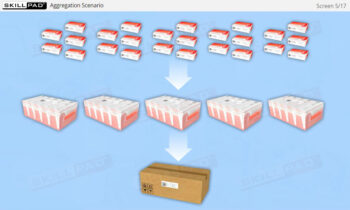
- Aggregation
- Authentication
- Compliance
- Counterfeiting
- Drug Quality and Security Act
- Drug Supply Chain Security Act (DSCSA)
- Packaging
- Pallet
- Product Recall
- Product Tracking
- Serialization
- Supply Chain
- Supply Chain Mapping
- Track and Trace
- Transaction History
- Transaction Information
- Transaction Statement
- Traceability
- Traceability
- Wholesalers
- 3PL
Module Features
Animations
Voice Over
Knowledge Checks
Assessments
SCORM/AICC compatible
Full Screen