SOPs in API Manufacturing
Explores the essential role of Standard Operating Procedures (SOPs) in Active Pharmaceutical Ingredient (API) manufacturing. It focuses on the purpose, structure, and importance of complying with Good Manufacturing Practices (GMP). Learners will understand key aspects of SOP usage, including version control, approval processes, and the rules that must be followed. The module provides practical knowledge on how to ensure consistency, safety, and product quality through the proper maintenance and execution of SOPs.
Whether for onboarding or annual refresher training, this Module is seamlessly deployable on any LMS and can be tailored to your company’s exact needs.
Duration: 30 Mins
Grade: Core Library
Description
- Understand the Purpose and Importance of SOPs: Understand the critical role SOPs play in ensuring consistent, compliant, and high-quality production processes in API manufacturing.
- Comply with GMP Regulations: Learn how to meet GMP requirements by following written, approved SOPs, reducing the risks associated with verbal or outdated instructions.
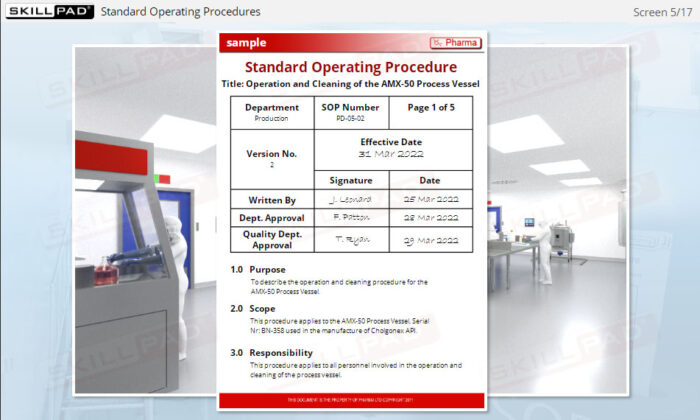
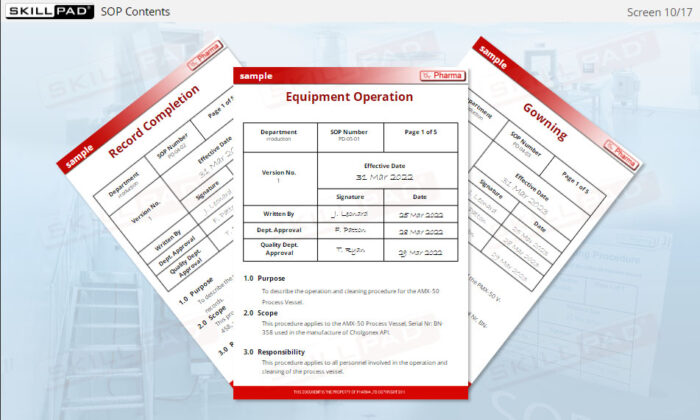
- Understand the Structure and Content of SOPs: Become familiar with the typical contents of an SOP, including key elements like task instructions, version control, and approval signatures.
- Manage SOP Version Control Effectively: Understand the importance of version control in keeping procedures current and valid, ensuring the correct SOP is always used during manufacturing tasks.
- Follow SOP Usage Rules: Learn the critical rules for using SOPs, including never altering procedures, always using the latest version, and avoiding shortcuts to maintain product quality and safety.
Learning Objectives
- Describe the purpose of Standard Operating Procedures (SOPs).
- Explain why SOPs are essential in API manufacturing.
- Explain the purpose of SOP version control.
- List the information typically contained in a GMP-compliant SOP.
- List the rules that must be followed when using SOPs.
Keywords
- API Manufacturing
- Approval Date
- Approval Signature
- Clean
- Contamination
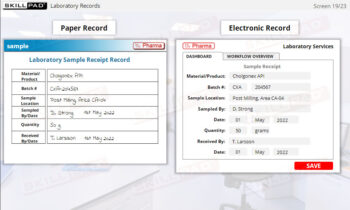
- Documentation
- Effective Date
- GMP Compliance
- GMP Regulations
- Instructional Procedures
- Manufacturing Process
- Operating Procedures
- Quality Control
- Quality Department
- Quality Manager
- Revision Number
- SOP Approval
- SOP Number
- SOP Usage
- SOP Version Control
- Task Instructions
- Training
- Unsafe Products
- Version Control
Module Features
Animations
Voice Over
Knowledge Checks
Assessments
SCORM/AICC compatible
Full Screen