SOPs in Finished Dose Manufacturing
An overview of the critical role of Standard Operating Procedures (SOPs) in finished dose manufacturing. This module explains the purpose of SOPs in ensuring compliance with Good Manufacturing Practices (GMP) and highlights their importance in maintaining consistency, quality control, and safety in production. Through scenarios and detailed guidance, learners will explore the essential elements of SOPs, including version control, content requirements, and strict adherence to written procedures to prevent errors and maintain product integrity.
Whether for onboarding or annual refresher training, this Module is seamlessly deployable on any LMS and can be tailored to your company’s exact needs.
Duration: 30 Mins
Grade: Core Library
Description
- Learn About the Purpose and Use of SOPs: Understand why SOPs are essential in finished dose manufacturing and how they support GMP compliance by standardizing processes to ensure consistent product quality.
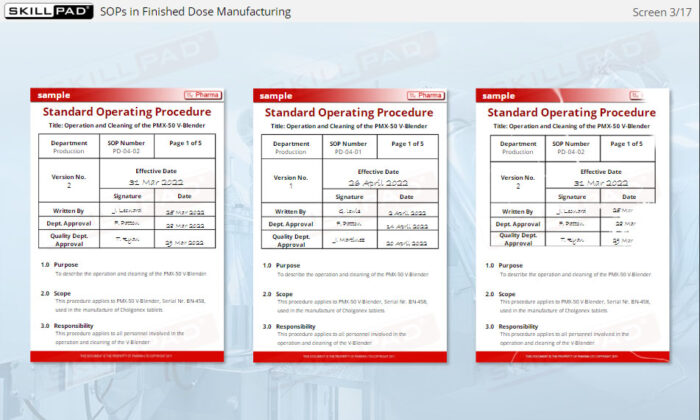
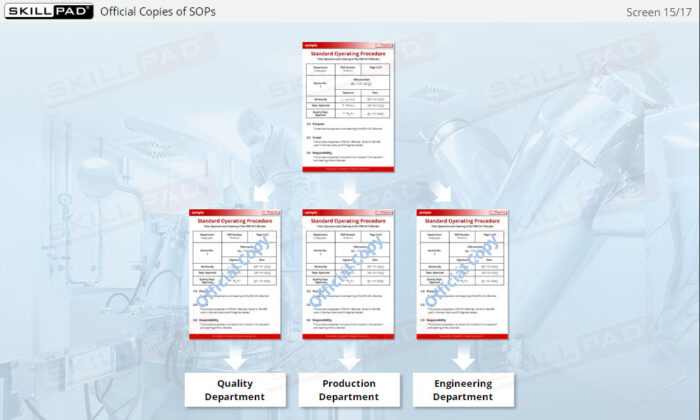
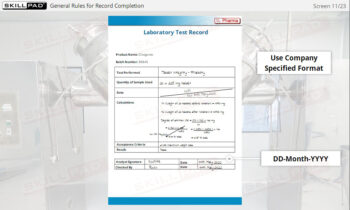
- Understand SOP Structure and Requirements: Learn about the components of a GMP-compliant SOP, including the significance of approval dates, version control, and proper documentation to meet regulatory standards.
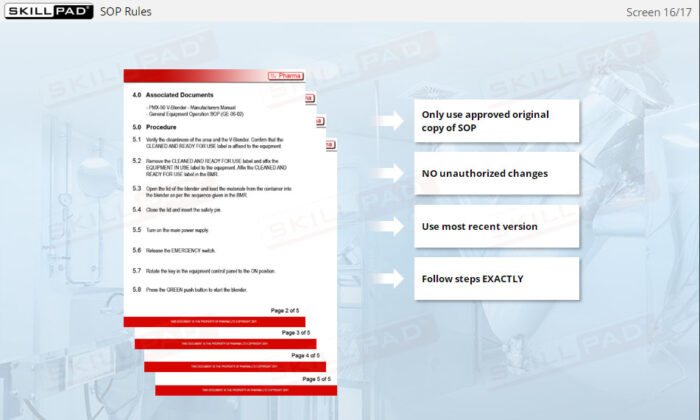
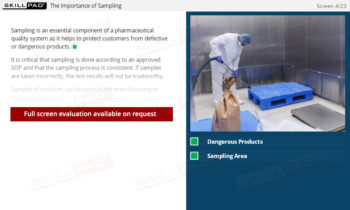
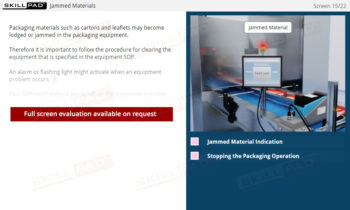
- Recognize the Importance of SOP Adherence: Understand the risks associated with deviating from SOPs, such as potential contamination and quality failures, and why strict adherence to the latest approved version is essential for safety.
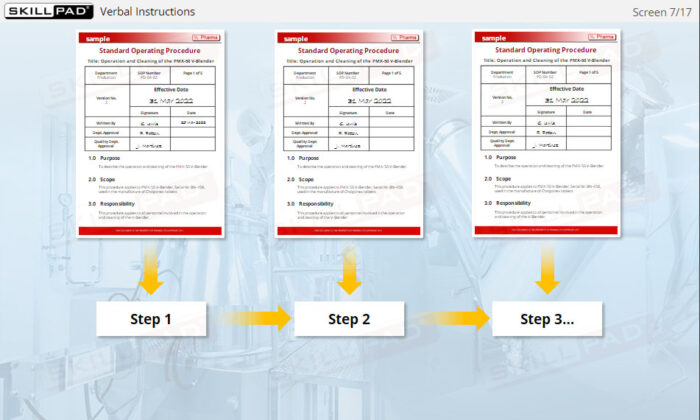
- Build Confidence in SOP Compliance: Understand the value of written procedures over verbal instructions, enabling trainees to follow SOPs accurately, reduce errors, and maintain regulatory compliance at every stage of the production process.
Learning Objectives
- Describe the purpose of Standard Operating Procedures (SOPs).
- Explain why SOPs are essential in Finished Dose Manufacturing.
- List the information typically contained in a GMP-compliant SOP.
- Explain the purpose of SOP version control.
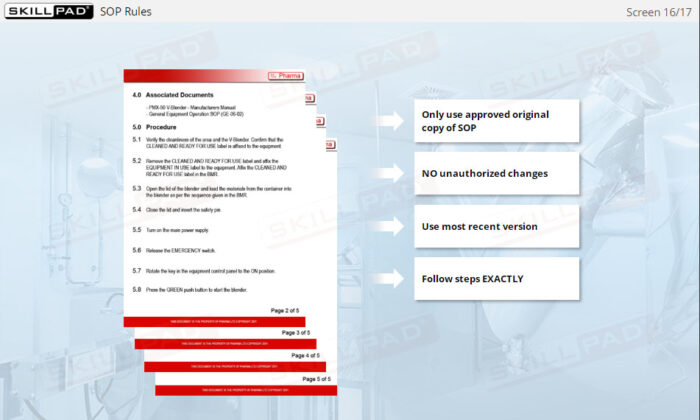
- List the rules that must be followed when using SOPs.
Keywords
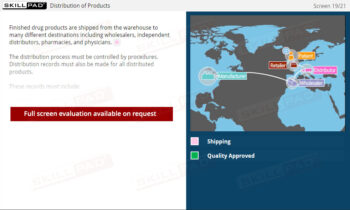
- Finished Dose Manufacturing
- GMP Compliance
- Good Manufacturing Practices
- Manufacturing Procedures
- Pharmaceutical Industry Training
- Pharmaceutical SOPs
- Regulatory Training
- SOPs In Manufacturing
- SOP Version Control
- Quality Assurance
Module Features
Animations
Voice Over
Knowledge Checks
Assessments
SCORM/AICC compatible
Full Screen