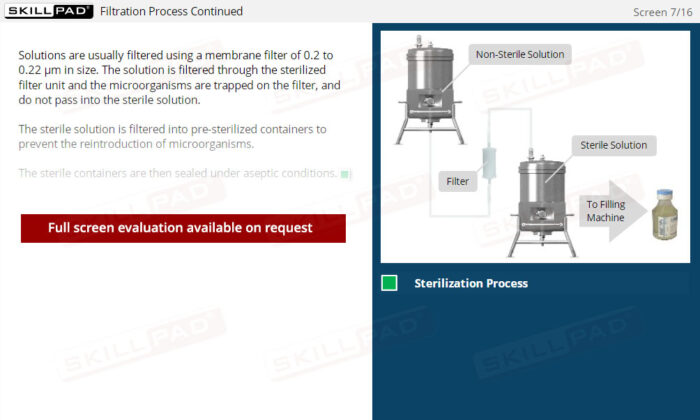
Sterile Filtration
An overview of sterile filtration in the pharmaceutical and biologics industries, explaining the principles and applications of this crucial sterilization method. It covers the design and assembly of filtration units, the stages of the filtration process, and the critical parameters required for successful sterile filtration. Learners will explore various filter integrity tests, including the Bubble Point Test, Pressure Decay Test, and Diffusive Airflow Test, all essential for ensuring the reliability and safety of the filtration process.
Part of Annex 1 training requirements.
Whether for onboarding or annual refresher training, this Module is seamlessly deployable on any LMS and can be tailored to your company’s exact needs.
Duration: 30 Mins
Grade: Core Library
Description
- Understand the Principles of Sterile Filtration: Understand how sterile filtration removes microorganisms using bacteria-proof filters to ensure product sterility in biologics and pharmaceutical manufacturing.
- Gain Knowledge of Filtration Equipment and Setup: Learn how to correctly assemble and handle filtration units, including selecting the appropriate filter types, sizes, and compatible housing units for effective sterilization.
- Understand Critical Process Parameters: Identify key factors that influence the filtration process, such as flow rate, filter assembly, and pressure control, and recognize the impact of deviations from these parameters.
- Perform Filter Integrity Testing: Become familiar with essential filter integrity tests like the Bubble Point Test, Pressure Decay Test, and Diffusive Airflow Test to verify the effectiveness and safety of filtration systems.
- Apply Aseptic Processing Best Practices: Learn aseptic techniques for handling and processing sterile solutions to minimize contamination risks throughout the filtration and packaging processes.
Learning Objectives
- Define what is meant by sterile filtration.
- List the uses of sterile filtration.
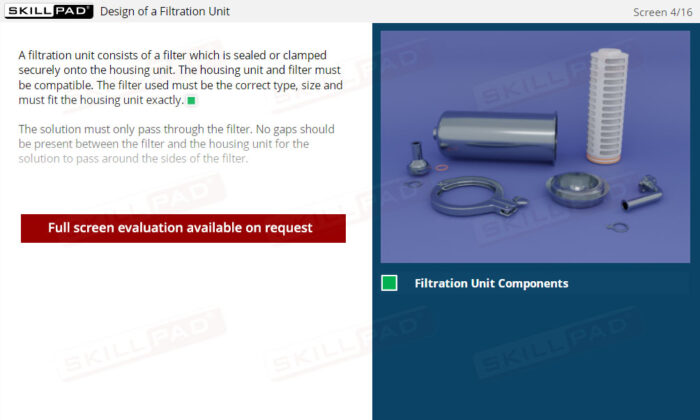
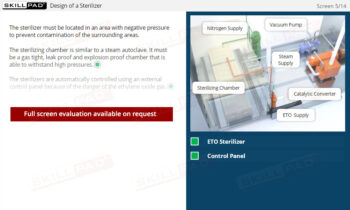
- Describe the design and assembly of a filtration unit.
- Identify the stages of the filtration process.
- List the critical parameters of sterile filtration.
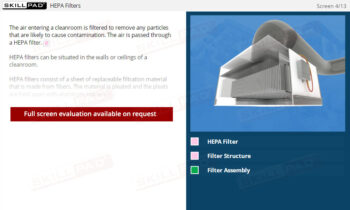
- Describe the different types of filter integrity tests.
Keywords
- Aseptic Conditions
- Batch Manufacturing Record (BMR)
- Biological Products
- Bubble Point Test
- Critical Parameters
- Diffusive Airflow Test
- Filtration Unit
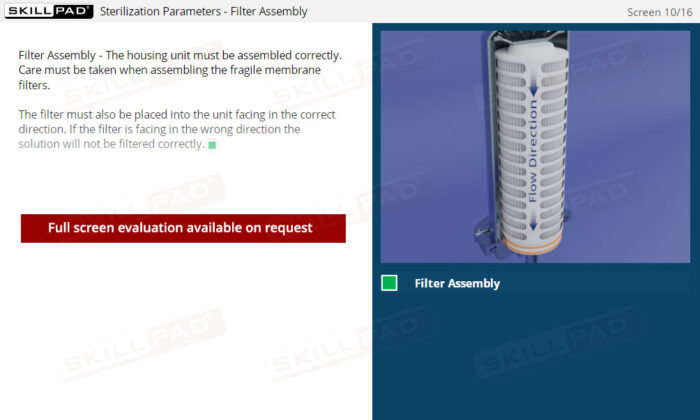
- Filter Assembly
- Filter Integrity Tests
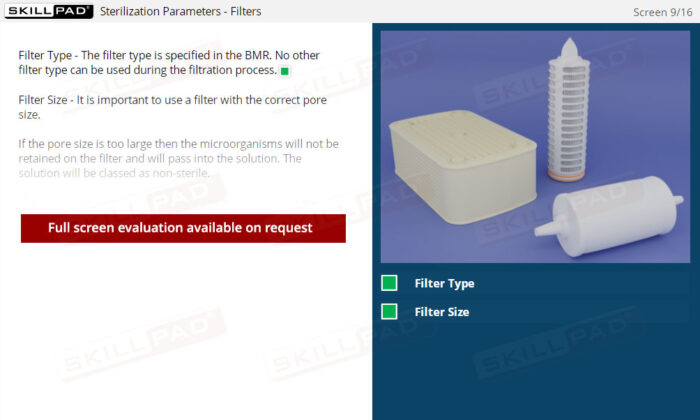
- Filter Type
- Filter Size
- Heat-Sensitive Injections
- Membrane Filters
- Microorganisms
- Pharmaceutical Manufacturing
- Pressure Decay Test
- Sterile Containers
- Sterile Filtration
- Sterilization Process
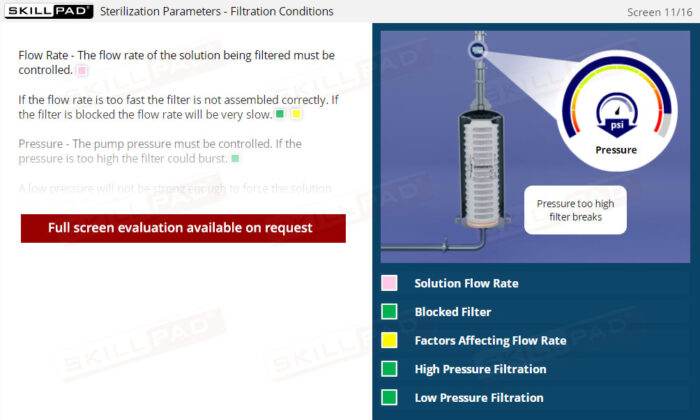
- Solution Flow Rate
- Solution Pressure
- Solution Sterilization
Module Features
Animations
Voice Over
Knowledge Checks
Assessments
SCORM/AICC compatible
Full Screen