Understanding Dissolution
Explore the fundamentals of dissolution testing and its critical role in the pharmaceutical industry. This module provides a detailed look at how a solid dosage drug form dissolves and is absorbed into the bloodstream, focusing on factors like polarity, temperature, pH, and agitation. Understand the principles of ‘in vivo’ and ‘in vitro’ dissolution analyses and learn about single-point and multi-point testing methods. Through animations and interactive explorations, grasp how dissolution testing ensures drug efficacy and compliance with regulatory standards.
Whether for onboarding or annual refresher training, this Module is seamlessly deployable on any LMS and can be tailored to your company’s exact needs.
Duration: 30 Mins
Grade: Core Library
Description
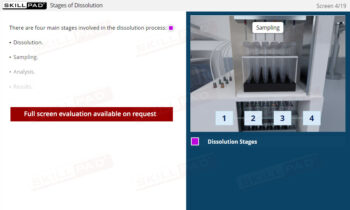
- Clear Comprehension of Dissolution Fundamentals: Master the definition and essential purpose of dissolution, including the physical and chemical processes that influence how drugs dissolve.
- Insight into Critical Testing Parameters: Recognize the factors impacting dissolution, such as solvent polarity, temperature, pH, and stirring, enhancing your understanding of formulation behavior in various environments.
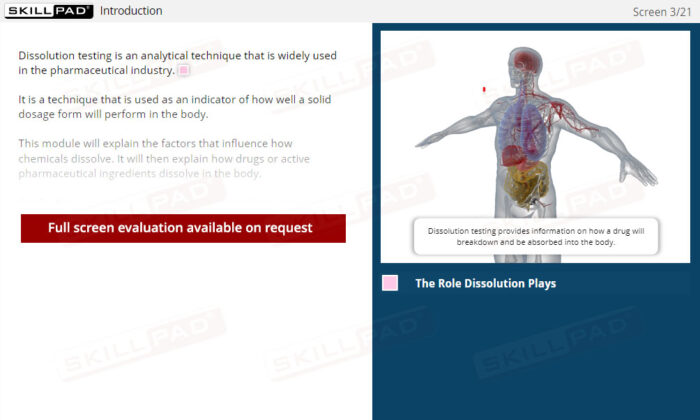
- Practical Understanding of Drug Absorption: Learn how a solid dosage form disintegrates and dissolves in the gastrointestinal tract before crossing into the bloodstream, crucial for assessing bioavailability.
- Differentiation Between Analytical Techniques: Distinguish between ‘in vivo’ and ‘in vitro’ testing, appreciating the importance of simulating human conditions in laboratory settings to predict drug performance.