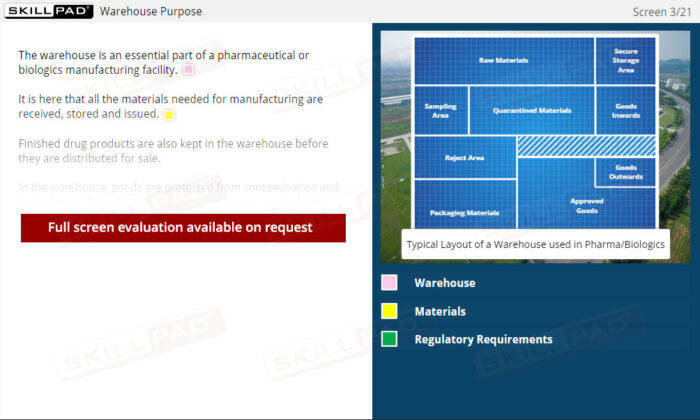
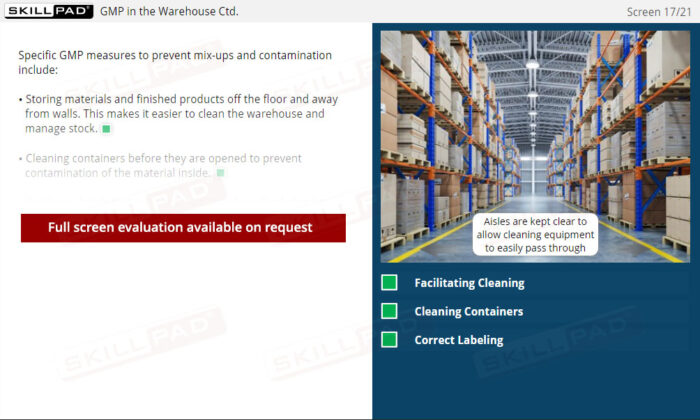
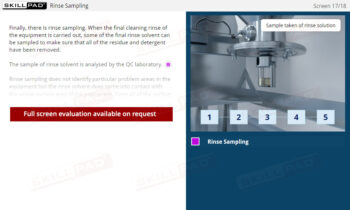
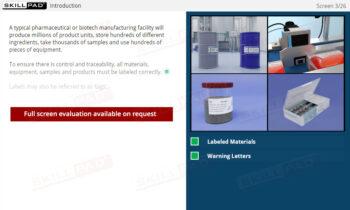
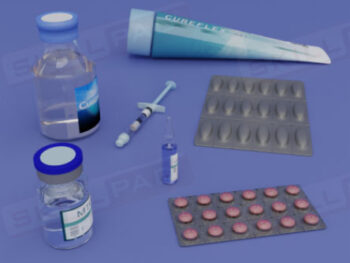
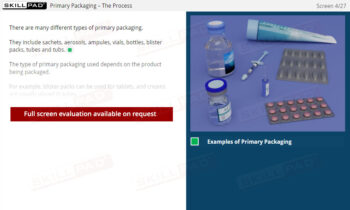
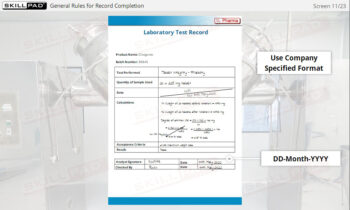
Warehousing
An overview of the essential functions of warehousing within pharmaceutical and biologics facilities, emphasizing its role in maintaining the quality, safety, and compliance of materials and products. This module explains core processes such as material receiving, storage conditions, issuance, and shipping, guided by ERP systems and strict GMP standards. Learners will gain an understanding of quality control status categories, warehouse SOPs, and the controlled conditions required for various products, including the specialized handling of rejected items and records for product distribution and recall.
Whether for onboarding or annual refresher training, this Module is seamlessly deployable on any LMS and can be tailored to your company’s exact needs.
Duration: 30 Mins
Grade: Core Library
Description
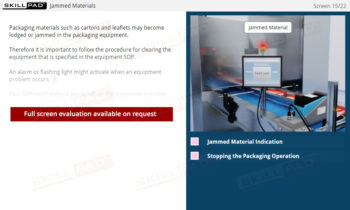
- Learn About Warehouse Functions: Gain foundational knowledge of the key functions of a pharmaceutical warehouse, including material receiving, stocking, issuing, and shipping, to support efficient operations and maintain product integrity.
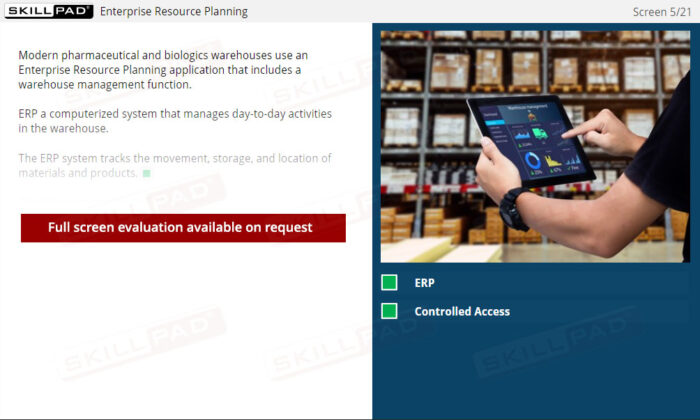
- Understand ERP and Inventory Control: Learn how ERP systems enhance warehouse management by tracking material movement, storage, and access, ensuring data accuracy and preventing unauthorized activity.
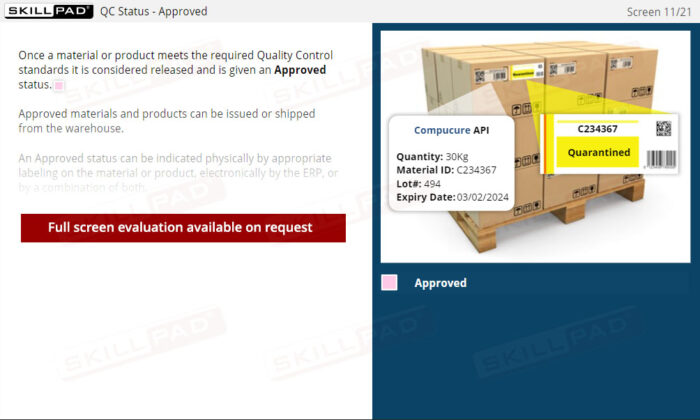
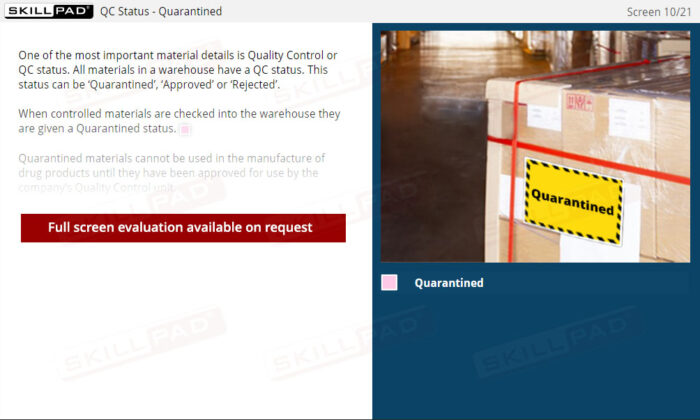
- Recognize Quality Control Status Categories: Understand the QC status categories—Quarantined, Approved, and Rejected—and how they determine material usage to meet stringent quality standards.
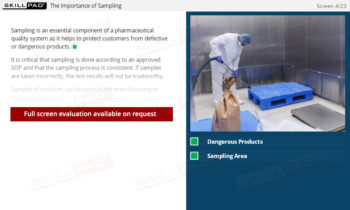
- Learn About GMP-Compliant Storage and Handling: Gain knowledge of controlled storage conditions, such as temperature, humidity, and security measures, to prevent contamination and ensure product stability.
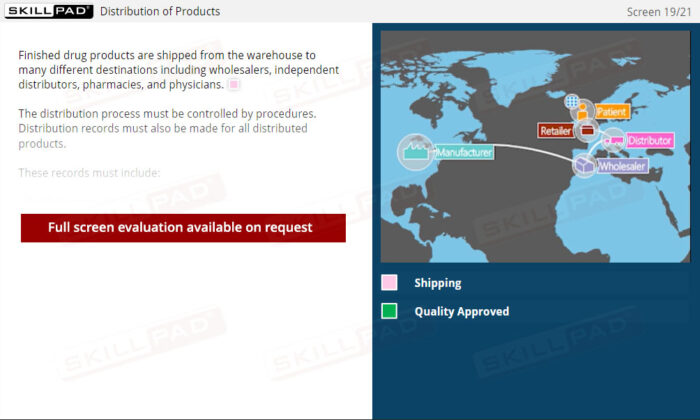
- Understand Distribution and Recall Procedures: Learn how to maintain precise distribution records, which are essential for traceability, efficient product recalls, and regulatory compliance in pharmaceutical distribution.
Learning Objectives
- Describe the purpose and functions of a warehouse.
- Describe the purpose of an Enterprise Resource Planning (ERP) System in relation to warehouse operations.
- Describe how materials are received into and issued out of a warehouse.
- List and explain the three QC status categories of materials and products in a warehouse.
- Name the warehouse storage conditions that must be controlled in a warehouse, and describe their importance.
- Explain the importance of product distribution records.
Keywords
- Drug Product Distribution
- ERP Systems in Pharmaceuticals
- GMP Compliance
- Material Handling
- Pharmaceutical Warehousing
- Quality Control Status
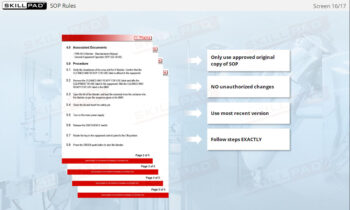
- SOP for Warehousing
- Traceability in Pharma
- Warehouse Operations
Module Features
Animations
Voice Over
Knowledge Checks
Assessments
SCORM/AICC compatible
Full Screen